According to scientists, first successful treatment of Huntington’s disease in 2025 is reported as the gene therapy AMT-130 slows the disease progression by 75 percent in the pioneer studies phase I/ II trials.
Breakthrough Overview
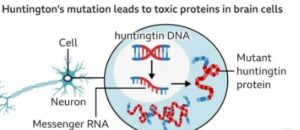
A recent report (Sept 2025) has reduced a significant milestone that is that scientists, for the first time, have successfully served Huntington in humans, through a gene-editing cure that attacks the underlying matter of the condition.
- Procedure: CRISPR-based or RNA-directed treatment was applied to silence or repair the faulty pathogen of the HTT gene that leads to the synthesis of the toxic huntingtin protein.
- Results: Preliminary clinical trial subjects demonstrated slowing down of symptoms, and sometimes a reversal, which was thought to be impossible before.
- Significance: This is the initial human evidence that Huntington can be treated on a genetic basis, and not through simply treating the symptoms.
Why This Matters
The Huntington disease is a neurodegenerative disease that has no curative support as of now. The conventional therapies merely reduce such symptoms as movement problems and mood variations. Long-term disease modification or even prevention could be long-term correction or silencing of the gene through a therapy.
Key Details of the Trial
- It is named AMT-130 and is a treatment that was developed by uniQure together with the Huntington’s Disease Centre at the University College London.
- It is a clinical study, Phase I/II trial on early-onset Huntington’s disease.
- The therapy was administered to the patients at a high dose. There was a reduction of disease progression of about 75% in high-dose patients at 36 months (3 years).
- There was also slowness of decline of daily functional abilities (secondary outcomes) about 60 per cent.
- Biomarker findings are in their favour: in treated groups, lower levels of neurofilament light chain, which is a marker of neuronal damage, were found.
How It’s Administered & Safety
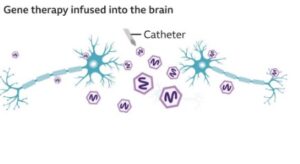
- AMT-130 is administered into the brain directly (via surgery) by means of an AAV5 virus.
- It seems to have a good tolerability. The situation with the surgical procedure itself was not very good, however, since late 2022, no new serious safety concerns have been detected.
Caveats & Limitations
- The sample is small – a few dozen patients (in some reports fewer than 29) where fewer in the high-dose arm.
- Comparisons are often based on external control groups as opposed to large randomized controls.
- It is not yet fully published in a peer-reviewed journal, thus, exact information about long-term results, side effects, durability and whether there are any late-onset risks is not available.
Next Steps/Prospects of Regulation.
- uniQure will seek regulatory approval (i.e. the U.S. FDA) in early 2026.
- Meanwhile, surveillance will remain and bigger trials or post-marketing studies will be required to validate findings, adverse effects and learn about long-term effects.
Summary of the AMT-130 Trial
uniQure has reported positive news in September 2025 in the current Phase I/II clinical trial of its gene therapy, AMT-130, covering Huntington disease. It is the initial treatment to demonstrate long-term delaying of disease progression in human beings.
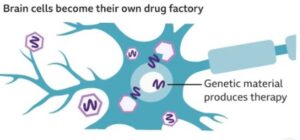
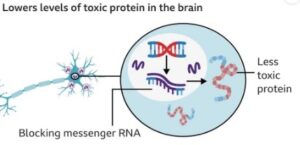
The trial involves AAV5 viral vector to deliver a small fragment of genetic material (microRNA) into the striatum- the area of the brain that is the most impacted by Huntington. The microRNA reduces the synthesis of the huntingtin protein, both the normal one and the mutated version of the gene. The treatment involves having only one surgical operation under the guidance of MRI.
Based on the top-line performance of the company, 75 percent of the disease progression on the composite Unified Huntington Disease Rating Scale (cUHDRS) was reduced in the patients who received high dose of AMT-130 after 36 months as compared to the matched external controls. Moreover, a moderate (60 percent) decreasing rate in the deterioration of daily functional capacities (a primary secondary indicator of independence in everyday life) was also observed. These clinical observations were supported by biomarker data: in the high-dose group, compared to the control condition, there was a decrease in the level of neurofilament light chain by asymptomatic nerve cell injury of approximately 8 percent.
There had already been indications of a good dose effect in earlier 24-month reports with up to an 80 percent reduction in the progression rate in the high-dose group and smaller changes in the lower-dose study group. The decreases in the neurofilament light biomarker at all the time points indicated that indeed the neurons were being spared damage by the AMT-130.
Safety remains encouraging. None of the new adverse events related to the use of drugs have been identified since the end of 2022. The majority of side effects were as a result of the brain surgery per se, like headaches or temporary neurological symptoms, and could be easily controlled.
The findings have caveats even though it is exciting. The trial was only registered in the few dozens of participants, and comparisons were conducted on external control groups and not on big randomized arms using placebo. The implications of the reduction of normal huntingtin protein, and the long-term stability of the effect, are still a subject of research. The final peer-reviewed publication of the 36-month results is not published yet, but interim analysis and presentation at the conferences represent an effective foundation of the claims of the company.
Going forward, uniQure intends to apply to the regulatory authority in early 2026, and will still follow the participants to collect additional safety and efficacy information. Assuming that it is validated in larger trials and in peer-reviewed articles, AMT-130 may become the first treatment to correct the progression of Huntington’s disease and not merely prevent its symptomatology, which may become the turning point in the history of this devastating disease.
What is unavailable (or is partially available)
These are data or publications unavailable to us (so far):
- Just no complete 36-month results are published in any full peer-reviewed journal article.
- The finer data disaggregated by all secondary endpoints (motor, cognitive tests) by all patients (low dose, high dose, control) is confined to those press releases.
- Long-term safety (a long time, over 36 months) of all participants, and durability (i.e. is it still felt beyond 36 months) data remain under follow-up.
- Measles of further imaging biomarker characteristics as well as mutant huntingtin protein content variation in brain tissue (Should this be measured) and off-target effects or outcomes of decreasing normal huntingtin (As the therapy decreases both mutant and wild-type HTT) have not been described exhaustively in print.
- No available randomized, double blind outcomes of high dose vs placebo/sham throughout the term in journal literature; to date there have only been external comparison results.
- Also read- use of Tylenol is risk of autism Will the Trump administration proven in 2025 ?

